Regulatory compliance is a decisive factor when selecting a máquina de remoção do cabelo laser de diódio for Europe and the United States. Clinics and distributors increasingly require proof that equipment meets diode laser hair removal machine CE FDA expectations.
FDA Oversight in the United States
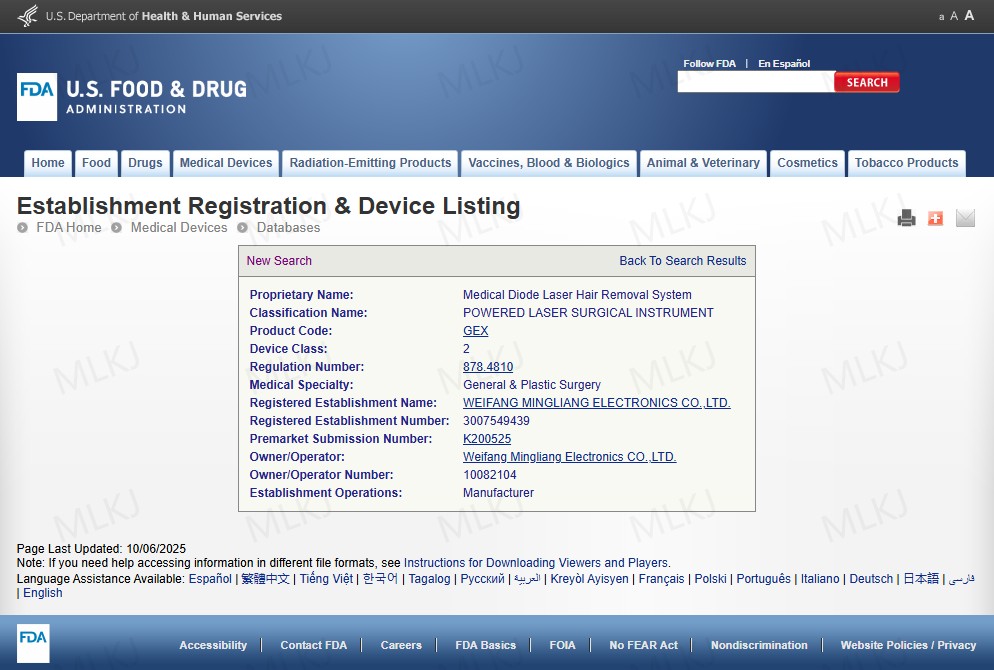
In the U.S., the FDA regulates dispositivo de remoção de cabelo laser platforms used in medical or aesthetic settings. Manufacturers must document safety and performance for laser machine hair removal professional applications.
https://www.fda.gov/medical-devices
EU MDR: What Clinics and Distributors Need to Know
Under the EU Medical Device Regulation (MDR), professional máquina de remoção de cabelo laser systems must demonstrate:
-
Risk management and clinical evaluation
-
Traceability and post-market surveillance
The European Commission details these obligations for medical and aesthetic laser devices.
https://health.ec.europa.eu/medical-devices-sector_en

Compliance and Professional Use
Compliance influences custo do equipamento de remoção de cabelo laser, but it also protects clinics sourcing diode laser hair removal machine for clinic operations. Clinical literature supports diode-based laser hair removal diode systems as suitable for professional use when compliant.
https://journals.lww.com/dermatologicsurgery

Perspectiva do Fabricante
With export experience to regulated markets, MLKJ structures documentation and quality systems to support MDR and FDA-aligned diode laser hair removal machine system deployment.
👉 Request CE / FDA Documentation
👉 Get Compliance Support
👉 Fabricante de Contacto
Para mais informações, por favor, contacte-nos





