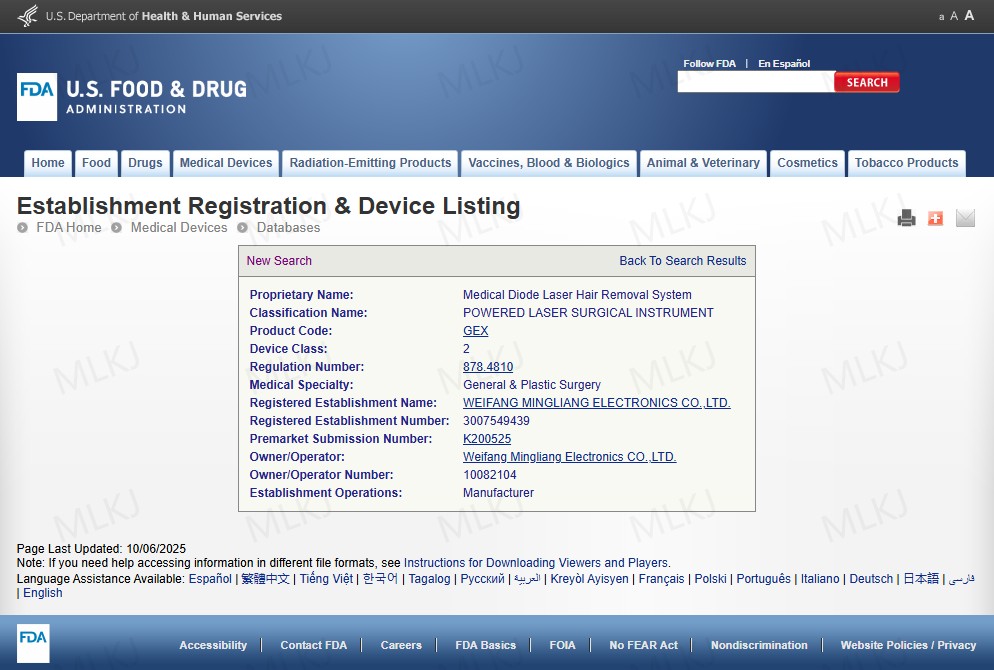
Regulatory compliance is a decisive factor when selecting a 半导体激光脱毛机 for Europe and the United States. Clinics and distributors increasingly require proof that equipment meets diode laser hair removal machine CE FDA expectations.
FDA Oversight in the United States
In the U.S., the FDA regulates 激光脱毛装置 platforms used in medical or aesthetic settings. Manufacturers must document safety and performance for laser machine hair removal professional applications.
https://www.fda.gov/medical-devices
EU MDR: What Clinics and Distributors Need to Know
Under the EU Medical Device Regulation (MDR), professional 激光脱毛机 systems must demonstrate:
-
Risk management and clinical evaluation
-
Traceability and post-market surveillance
The European Commission details these obligations for medical and aesthetic laser devices.
https://health.ec.europa.eu/medical-devices-sector_en
Compliance and Professional Use
Compliance influences 激光脱毛设备成本, but it also protects clinics sourcing diode laser hair removal machine for clinic operations. Clinical literature supports diode-based laser hair removal diode systems as suitable for professional use when compliant.
https://journals.lww.com/dermatologicsurgery
制造商视角
With export experience to regulated markets, MLKJ structures documentation and quality systems to support MDR and FDA-aligned diode laser hair removal machine system deployment.
👉 Request CE / FDA Documentation
👉 Get Compliance Support
👉 联系制造商
如需更多信息,请联系我们86 15965364558